The continued evaluation of the PFA-100

In the 1970-90’s a common preoperative evaluation included complete blood count (CBC), coagulation screening tests such as the prothrombin time (PT) and activated partial thromboplastin time (APTT). While the CBC provided platelet (PLT) counts, there was no assessment of their function. Frequently included was a test called the bleeding time (BT), which was allegedly used for screening for PLT function defects. The Ivy bleeding time entails making one or two uniform incisions along the forearm, while a blood pressure cuff is maintained at 40mmHg pressure. After incisions, the blood seeping from the wound is absorbed via filter paper until the incision stops bleeding. For children, the Duke bleeding time was typically employed by making an incision on the ear lobe (without blood pressure cuff). The typical normal Ivy BT was ~ 7-9 minutes, while a normal Duke BT was ~5mins. This was considered an acceptable means for assessing platelet dysfunction, and commonly used in certain populations that have higher bleeding risk (e.g. renal biopsy). In 1991, a published meta-analysis review by Lind1, and another by Rodgers and Levin2 demonstrated the lack of relationship between BT results and bleeding outcomes. Those reports, along with changes in the US law for testing performance (BT testing was performed by any laboratory personnel, then with 1988 Clinical and Laboratory Improvement Acts (CLIA), the BT was considered to high complexity and only medical technologists were the appropriate laboratory personnel to perform the BT), there became a gradual movement away from using the BT as a means of screening for PLT dysfunction.
However, the clinical staff was not comforted by the lack of replacement testing, if the BT was removed from the laboratory testing menu. Coincidentally, in 1995 Kundu and colleagues described a unique analyzer, the PFA-100 (Platelet Function Analyzer-100; Dade International) that appeared to be sensitive for platelet defects.3 In the same journal edition, Mammen et al described some preliminary field study data which suggested that the PFA-100 detected primary hemostasis defects (aka PLT function defects) than the BT.4 The subsequent data that was submitted to the FDA was published in 1998 by Mammen and collaborators.5 The study entailed the comparison of adult (>18 years of age) normal donors, adults known vonWillebrand disease (VWD) patients, and adults with other known PLT defects (e.g. Glanzmann’s thrombasthenia, GT), and a paired testing of adult normal donors pre/post single dose of 325mg aspirin (ASA). Each patient collection (except post ASA sampling) had traditional platelet aggregometry, vnoWillebrand factor testing panel consisting of PT, APTT, BT, Fibrinogen (FBG), factor VIII (F8), ristocetin cofactor (VWF:RCo), von Willebrand antigen (VWF:Ag), von Willebrand multimeric analysis, CBC, and PFA-100 using the dual cartridge system consisting of Collagen/ADP (CADP) collagen epinephrine (CEPI). For post-ASA, only platelet aggregation and PFA-100 testing were performed within 2-30 hours after ASA ingestion. This study demonstrated that for the 44 patients with known PLT dysfunction, the PFA-100 was more sensitive than the BT (95.5% versus 59.1% respectively). With the PFA-100, there was now a potential to evaluate for PLT dysfunction rapidly, cheaply, and can be performed at any institution with little technical or interpretative expertise.
Note that this study, which was the basis of FDA approval of this device, only evaluated patients with known PLT function defects (VWD and GT) and ASA effect. However, since the PFA-100 availability, there has been numerous publications about the use of the PFA-100 outside the initial intended use of this device. Most notably are studies evaluating ASA resistance, other anti-PLT drug (e.g. clopidigrel) effect, and other populations (e.g. uremic, pediatric). The purpose of this document is to 1) review the PFA-100 principles of operation, 2) consider the preanalytical variables that may or may not impact the PFA-100 results, 3) describe the current literature of recommended use of this test. A newer version of the PFA-100, the PFA-200 is not available in the US. The primary difference between the devices, aside from aesthetics (appearance), bar code scanner, and other features, is a third cartridge (INNOVANCE PFA P2Y) to be detailed later.
PFA-100 Principle of operation:
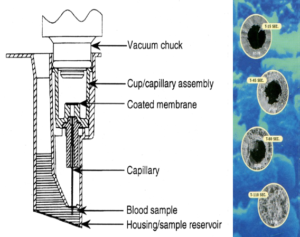
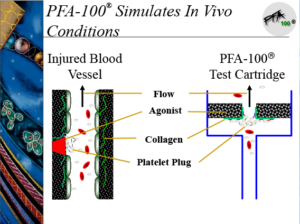
The PFA-100 testing system consists of the instrument and dual set of cartridges (CEPI and CADP). Whole blood (~800µL) is placed into the sample reservoir of the cartridges (Figure 1), and the samples are introduced into the instrument. Upon initiation, a chuck is placed over the cartridge, and a constant vacuum is applied so that the blood travels from the holding area up through a capillary and through a micropore that contains either CEPI or CADP. The blood continues to travel through the capillary until the micropore is occluded. (Figure 1) The occlusion of the micropore results in a pressure change that is recorded by the instrument as a “closure time” (CT). The in-vitro cartridge with shear rate conditions mimics capillary flow in-vivo. (figure 2)
Typical normal (reference interval, RI) closure times for CPI are ~60-200 seconds and for CADP ~70-120 seconds6 with the RI being affected by citrate concentration and collection method.7
Preanalytical Variables (PAV) that affect PFA-100 testing:
Aside from citrate concentration, there are several physiological and analytical variables that may affect PLT function testing:
Physiological variables: oral contraceptive and smoking were not significant variables associated with PFA-100 testing.8 Circadian variation was noted9,10, as well as individuals with higher body mass index (BMI)10 Gender was not significantly different in one study8, but significantly different in another.10 Ovarian cycle was noted to have decreased CEPI CT in the luteal phase as compared to the follicular phase, with no significant difference seen with CADP.11,12 Oral contraceptives (OC) had some impact on PFA-100 closure times related to type of OC.12
The PFA-100 closure times are primarily influenced by PLT (count or dysfunction) and von Willebrand factor (VWF). 5,13,14 As blood type O is associated with lower VWF levels, type O individuals have higher PFA-100 CT.13,14 Anemia and thrombocytopenia are known to affect PFA-100 CT, with package insert describing hematocrits of <35% and PLT counts of <150,000/mm3 as thresholds.15 There is no effect of FBG, F8 or factor IX (F9) deficiency (Hemophilia A and B respectively) and PFA-100 CTs.3,13
For CEPI, cord blood collected samples had shorter PFA CTs than neonatal CTs, and neonatal CTs had longer CTs than adult CTs, with children essentially having same CTs as adults.16,17 For CADP, cord blood samples had shorter CTs than neonatal samples, which were both shorter than adult CTs16, which were not attributed to the cellular components of these groups, and most likely secondary to high levels of VWF.18
There will be dietary and drug effects on the PFA-100 test. Some are anticipated, such as ASA or other NSAIDs, some unanticipated such as cocoa19,20, red wine21, and other potential foods, herbal supplements or fish oil derivatives, prescription/in-hospital drugs15 or over-the-counter (OTC) drugs such antihistamines. The cocoa effect was a happenstance observation. When the PFA-100 was first approved for use in the US, it was required that a normal quality control (QC) sample be tested each day of operation. As we had a local blood donor center just a mile from the university and they provided a normal donor sample every weekday for QC use. However, ~40% of the time, the PFA CTs were abnormal, despite normal CBC and classified “normal donor”. As blood donor centers recommend donors to eat before donating blood, without dietary restriction, we soon discovered that chocolate milk consumption prior to blood collection prolonged the PFA CTs, as well as relatively recent ASA intake.22 The daily “wet control” QC requirement has been replaced by an instrument electronic check.
Ex-vivo variables: Prolonged tourniquet times for blood collection may release VWF stored in the Weibel-Palade bodies of the endothelium, thereby masking a mild VWF deficiency. Although needle gauge (21g vs 23g) did not affect PFA-100 testing17, citrate concentration and collection type (vacuum versus syringe) did demonstrate differences.7,23 In a single site study, the authors claim no difference between PFA-100 CTs when collected from venous, arterial, central venous line or standard venous blood collection, but the provided graphs did show significant (normal vs abnormal) differences in some collection techniques.24 Poorly collected samples that cause hemolysis or platelet clumping will cause errors (“Flow Obstruction”) in the instrument operation.25 There was no effect of heparin or PPACK on PFA-100 CTs26, but pneumatic tube transportation demonstrated a slight prolongation of the PFA-100 CTs.27 Sample stability of the PFA-100 samples are noted to be 4 hours at room temperature.15
Clinical Utility of the PFA-100
Due to the ease of use and rapid time to results (within 15mins) the introduction of the PFA-100 has created an opportunity to evaluate PLT function in a variety of settings. The vast majority of publications attempt to utilize the PFA-100 for assessing ASA or other anti-PLT drugs (particularly P2Y12 drugs that are ADP receptor inhibitors) “resistance”, although the term “resistance” is poorly defined. The PFA-200 P2Y12 cartridge (unavailable in the US) has been demonstrated to be equivalent to other rapid devices (e.g. VerifyNow) for assessing these class of drugs.28,29, but may be anticoagulant dependent.30 The PFA-100 has been found to be useful, albeit in a small or single series of preeclamptic pregnant women31, menorrhagia32, primary thrombocythemia33,34, predicting transfusion requirements in aortic valve replacement35, IIb-IIIa blockers in stent placement36, trauma37, and even for QC purposes for PLT components.38 The PFA-100 has not been proven to be reliable for assessing ASA resistance39 or associated with transient ischemic attack or stroke.40
As a reminder, the initial evaluation of the PFA-100 was in patients with known PLT dysfunction and known ASA ingestion. As the PFA-100 method does NOT adequately screen for all PLT dysfunctions, such as storage pool or release defects41,42, or rare milder PLT defects (e.g. Hermansky Pudlak syndrome43) or mild VWD43,44, and thus is not recommended as a general screening method for all PLT dysfunction.45,46
However, the PFA-100 has been shown to adequately detect moderate to severe VWD and GT, and thus normal PFA-100 CT will rule out those diseases.5,6,13,14,46,47 Additionally, the PFA-100 has been shown to be useful in assessing desmopressin (DDAVP) response in VWD treatment, with variable to no response when using VWF replacements products such as Humate P.47-50
The potential PFA CT results are either 1) normal (within the RI), 2) prolonged (higher CT than the RI), or decreased (shorter CTs than the RI):
| PFA 100 CEPI | PFA 100 CADP | |
| Normal functioning PLTs, milder PLT disorders, post VWD treatment, dietary effect | Within RI to slightly prolonged CTs | Within RI to slightly prolonged CTs |
| Moderate to severe PLT disorders or VWD, drug or dietary effect | Slightly to markedly prolonged CTs | Slightly to markedly prolonged CTs |
| Activated PLTs or increased circulating VWF levels | Within RI to decreased CTs | Within RI to decreased CTs |
Conclusions
The continued evaluation of the PFA-100 is non-traditional settings will most likely continue given the ease of assessing PLT function with this device. However, while significantly better than the BT and while the PFA-100 demonstrates good sensitivity, the lack of specificity may be problematic for those patients that fall outside its intended use. The judicious use of the PFA-100 should be encouraged.
References
- Lind SE. The bleeding time does not predict surgical bleeding. Blood. 1991;77(12):2547-52.
- Rodgers RP, Levin J. Bleeding time revisited. Blood. 1992;79(9):2495-7.
- Kundu SK, Heilmann EJ, Sio R, Garcia C, Davidson RM, Ostgaard RA. Description of an in vitro platelet function analyzer–PFA-100. Semin Thromb Hemost. 1995;21 Suppl 2:106-12.
- Mammen EF, Alshameeri RS, Comp PC. Preliminary data from a field trial of the PFA-100 system. Semin Thromb Hemost. 1995;21 Suppl 2:113-21.
- Mammen EF, Comp PC, Gosselin R, Greenberg C, Hoots WK, Kessler CM, Larkin EC, Liles D, Nugent DJ. PFA-100 system: a new method for assessment of platelet dysfunction. Semin Thromb Hemost. 1998;24(2):195-202.
- Favaloro EJ. Clinical utility of the PFA-100. Semin Thromb Hemost. 2008;34(8):709-33. doi: 10.1055/s-0029-1145254.
- Heilmann EJ, Kundu SK, Sio R, Garcia C, Gomez R, Christie DJ. Comparison of four commercial citrate blood collection systems for platelet function analysis by the PFA-100 system. Thromb Res. 1997;87(1):159-64.
- Böck M, De Haan J, Beck KH, Gutensohn K, Hertfelder HJ, Karger R, Heim MU, Beeser H, Weber D, Kretschmer V. Standardization of the PFA-100(R) platelet function test in 105 mmol/l buffered citrate: effect of gender, smoking, and oral contraceptives. Br J Haematol. 1999;106(4):898-904.
- Feuring M, Wehling M, Ruf A, Schultz A. Circadian variation of platelet function measured with the PFA-100. Platelets. 2009;20(7):466-70. doi:10.3109/09537100903226034.
- Haubelt H, Anders C, Vogt A, Hoerdt P, Seyfert UT, Hellstern P. Variables influencing Platelet Function Analyzer-100 closure times in healthy individuals. Br J Haematol. 2005;130(5):759-67.
- Feuring M, Christ M, Roell A, Schueller P, Losel R, Dempfle CE, Schultz A, Wehling M. Alterations in platelet function during the ovarian cycle. Blood Coagul Fibrinolysis. 2002;13(5):443-7. PubMed PMID: 12138372.
- Roell A, Schueller P, Schultz A, Losel R, Wehling M, Christ M, Feuring M. Effect of oral contraceptives and ovarian cycle on platelet function. Platelets. 2007;18(2):165-70. PubMed PMID: 17365866.
- Fressinaud E, Veyradier A, Truchaud F, Martin I, Boyer-Neumann C, Trossaert M, Meyer D. Screening for von Willebrand disease with a new analyzer using high shear stress: a study of 60 cases. Blood. 1998;91(4):1325-31.
- Ardillon L, Ternisien C, Fouassier M, Sigaud M, Lefrançois A, Pacault M, Ribeyrol O, Fressinaud E, Boisseau P, Trossaërt M. Platelet function analyser (PFA-100) results and von Willebrand factor deficiency: a 16-year ‘real-world’ experience. Haemophilia. 2015;21(5):646-52. doi: 10.1111/hae.12653.
- Dade® PFA Collagen/EPI Test Cartridge COLL EPI CARTRIDGE Dade® PFA Collagen/ADP Test Cartridge COLL ADP CARTRIDGE package inserts. Siemens Healthcare, Tarrytown, NY. B4170G20AU11 Rev. 05.
- Saxonhouse MA, Garner R, Mammel L, Li Q, Muller KE, Greywoode J, Miller C, Sola-Visner M. Closure times measured by the platelet function analyzer PFA-100 are longer in neonatal blood compared to cord blood samples. Neonatology. 2010;97(3):242-9. doi: 10.1159/000253755.
- Carcao MD, Blanchette VS, Dean JA, He L, Kern MA, Stain AM, Sparling CR, Stephens D, Ryan G, Freedman J, Rand ML. The Platelet Function Analyzer (PFA-100): a novel in-vitro system for evaluation of primary haemostasis in children. Br J Haematol. 1998;101(1):70-3.
- Roschitz B, Sudi K, Köstenberger M, Muntean W. Shorter PFA-100 closure times in neonates than in adults: role of red cells, white cells, platelets and von Willebrand factor. Acta Paediatr. 2001;90(6):664-70.
- Pearson DA, Paglieroni TG, Rein D, Wun T, Schramm DD, Wang JF, Holt RR, Gosselin R, Schmitz HH, Keen CL. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002;106(4-5):191-7.
- Rein D, Paglieroni TG, Wun T, Pearson DA, Schmitz HH, Gosselin R, Keen CL. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72(1):30-5.
- Rein D, Paglieroni TG, Pearson DA, Wun T, Schmitz HH, Gosselin R, Keen CL. Cocoa and wine polyphenols modulate platelet activation and function. J Nutr. 2000;130(8S Suppl):2120S-6S. doi: 10.1093/jn/130.8.2120S.
- Paglieroni TG, Janatpour K, Gosselin R, Crocker V, Dwyre DM, MacKenzie MR, Holland PV, Larkin EC. Platelet function abnormalities in qualified whole-blood donors: effects of medication and recent food intake. Vox Sang. 2004;86(1):48-53.
- Lippi G, Ippolito L, Zobbi V, Sandei F, Favaloro EJ. Sample collection and platelet function testing: influence of vacuum or aspiration principle on PFA-100 test results. Blood Coagul Fibrinolysis. 2013;24(6):666-9. doi:10.1097/MBC.0b013e32835fada7.
- Lancé MD, Henskens YM, Nelemans P, Theunissen MH, Oerle RV, Spronk HM, Marcus MA. Do blood collection methods influence whole-blood platelet function analysis? Platelets. 2013;24(4):275-81. doi: 10.3109/09537104.2012.689038.
- Lippi G, Fontana R, Avanzini P, Aloe R, Ippolito L, Sandei F, Favaloro EJ. Influence of mechanical trauma of blood and hemolysis on PFA-100 testing. Blood Coagul Fibrinolysis. 2012;23(1):82-6. doi: 10.1097/MBC.0b013e32834c6cb5.
- Kottke-Marchant K, Powers JB, Brooks L, Kundu S, Christie DJ. The effect of antiplatelet drugs, heparin, and preanalytical variables on platelet function detected by the platelet function analyzer (PFA-100). Clin Appl Thromb Hemost. 1999;5(2):122-30.
- Dyszkiewicz-Korpanty A, Quinton R, Yassine J, Sarode R. The effect of a pneumatic tube transport system on PFA-100 trade mark closure time and whole blood platelet aggregation. J Thromb Haemost. 2004;2(2):354-6.
- Linnemann B, Schwonberg J, Rechner AR, Mani H, Lindhoff-Last E. Assessment of clopidogrel non-response by the PFA-100 system using the new test cartridge INNOVANCE PFA P2Y. Ann Hematol. 2010;89(6):597-605. doi:10.1007/s00277-009-0881-9.
- Koessler J, Kobsar AL, Rajkovic MS, Schafer A, Flierl U, Pfoertsch S, Bauersachs J, Steigerwald U, Rechner AR, Walter U. The new INNOVANCE® PFA P2Y cartridge is sensitive to the detection of the P2Y₁₂ receptor inhibition. Platelets. 2011;22(1):20-7. doi: 10.3109/09537104.2010.514967.
- Edwards A, Jakubowski JA, Rechner AR, Sugidachi A, Harrison P. Evaluation of the INNOVANCE PFA P2Y test cartridge: sensitivity to P2Y(12) blockade and influence of anticoagulant. Platelets. 2012;23(2):106-15. doi:10.3109/09537104.2011.601361.
- Davies JR, Fernando R, Hallworth SP. Hemostatic function in healthy pregnant and preeclamptic women: an assessment using the platelet function analyzer (PFA-100) and thromboelastograph. Anesth Analg. 2007;104(2):416-20.
- Acharya S, Barraclough J, Ibrahim MS, Oxby C, Jones SE, Parapia L, O’donovan P. The usefulness of the platelet function analyser (PFA-100) in screening for underlying bleeding disorders in women with menorrhagia. J Obstet Gynaecol. 2008;28(3):310-4. doi: 10.1080/01443610802141910.
- Cesar JM, de Miguel D, García Avello A, Burgaleta C. Platelet dysfunction in primary thrombocythemia using the platelet function analyzer, PFA-100. Am J Clin Pathol. 2005;123(5):772-7.
- Tsantes AE, Dimoula A, Bonovas S, Mantzios G, Tsirigotis P, Zoi K, Kalamara E, Kardoulaki A, Sitaras N, Travlou A, Dervenoulas J, Vaiopoulos G. The role of the Platelet Function Analyzer (PFA)-100 and platelet aggregometry in the differentiation of essential thrombocythemia from reactive thrombocytosis. Thromb Res. 2010;125(2):142-6. doi: 10.1016/j.thromres.2009.06.030.
- Sucker C, Litmathe J, Feindt P, Zotz R. Platelet function analyzer (PFA-100) as a useful tool for the prediction of transfusion requirements during aortic valve replacement. Thorac Cardiovasc Surg. 2011;59(4):233-6. doi:10.1055/s-0030-1250375.
- Van der Planken MG, Claeys MJ, Vertessen FJ, Dilling D, Bosmans JM, Berneman ZN, Michiels JJ, Vrints C. Comparison of turbidimetric aggregation and in vitro bleeding time (PFA-100) for monitoring the platelet inhibitory profile of antiplatelet agents in patients undergoing stent implantation. Thromb Res. 2003;111(3):159-64.
- Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma. 2001;51(4):639-47.
- Beck KH. Quality control of platelets during storage by the PFA-100: a comparison to platelet aggregation. Transfus Apher Sci. 2002;27(3):247-53.
- Kovács EG, Katona É, Bereczky Z, Homoródi N, Balogh L, Tóth E, Péterfy H, Kiss RG, Édes I, Muszbek L. Evaluation of laboratory methods routinely used to detect the effect of aspirin against new reference methods. Thromb Res. 2014;133(5):811-6. doi: 10.1016/j.thromres.2013.10.008.
- Lim ST, Coughlan CA, Murphy SJ, Fernandez-Cadenas I, Montaner J, Thijs V, Marquardt L, McCabe DJ. Platelet function testing in transient ischaemic attack and ischaemic stroke: A comprehensive systematic review of the literature. Platelets. 2015;26(5):402-12. doi: 10.3109/09537104.2015.1049139.
- The PFA-100® does not predict delta-granule platelet storage pool deficiencies. Sladky JL, Klima J, Grooms L, Kerlin BA, O’Brien SH. Haemophilia. 2012;18(4):626-9. doi: 10.1111/j.1365-2516.2011.02733.x.
- Cattaneo M, Lecchi A, Agati B, Lombardi R, Zighetti ML. Evaluation of platelet function with the PFA-100 system in patients with congenital defects of platelet secretion. Thromb Res. 1999;96(3):213-7.
- Harrison P, Robinson M, Liesner R, Khair K, Cohen H, Mackie I, Machin S. The PFA-100: a potential rapid screening tool for the assessment of platelet dysfunction. Clin Lab Haematol. 2002;24(4):225-32.
- Castaman G, Tosetto A, Goodeve A, Federici AB, Lethagen S, Budde U, Batlle J, Meyer D, Mazurier C, Goudemand J, Eikenboom J, Schneppenheim R, Ingerslev J, Habart D, Hill F, Peake I, Rodeghiero F. The impact of bleeding history, von Willebrand factor and PFA-100(®) on the diagnosis of type 1 von Willebrand disease: results from the European study MCMDM-1VWD. Br J Haematol. 2010;151(3):245-51. doi: 10.1111/j.1365-2141.2010.08333.x.
- Hayward CP, Harrison P, Cattaneo M, Ortel TL, Rao AK; Platelet Physiology Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Platelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet function. J Thromb Haemost. 2006;4(2):312-9.
- Podda GM, Bucciarelli P, Lussana F, Lecchi A, Cattaneo M. Usefulness of PFA-100 testing in the diagnostic screening of patients with suspected abnormalities of hemostasis: comparison with the bleeding time. J Thromb Haemost. 2007;5(12):2393-8.
- Harrison P. The role of PFA-100 testing in the investigation and management of haemostatic defects in children and adults. Br J Haematol. 2005;130(1):3-10.
- Favaloro EJ. Laboratory monitoring of therapy in von Willebrand disease: efficacy of the PFA-100 and von Willebrand factor:collagen-binding activity as coupled strategies. Semin Thromb Hemost. 2006;32(6):566-76.
- Cattaneo M, Federici AB, Lecchi A, Agati B, Lombardi R, Stabile F, Bucciarelli P. Evaluation of the PFA-100 system in the diagnosis and therapeutic monitoring of patients with von Willebrand disease. Thromb Haemost. 1999;82(1):35-9.
- Favaloro EJ, Lloyd J, Rowell J, Baker R, Rickard K, Kershaw G, Street A, Scarff K, Barrese G, Maher D, McLachlan AJ. Comparison of the pharmacokinetics of two von Willebrand factor concentrates [Biostate and AHF (High Purity)] in people with von Willebrand disorder. A randomised cross-over, multi-centre study. Thromb Haemost. 2007;97(6):922-30.